Orthocell (ASX:OCC) has secured $17m in a recent capital raising initiative, aimed at funding the US launch of its flagship Remplir device. The funds will also support expansion into Singapore, Southeast Asia, and Europe, and be allocated to scaling up manufacturing infrastructure and enhancing cost efficiency through automation projects.
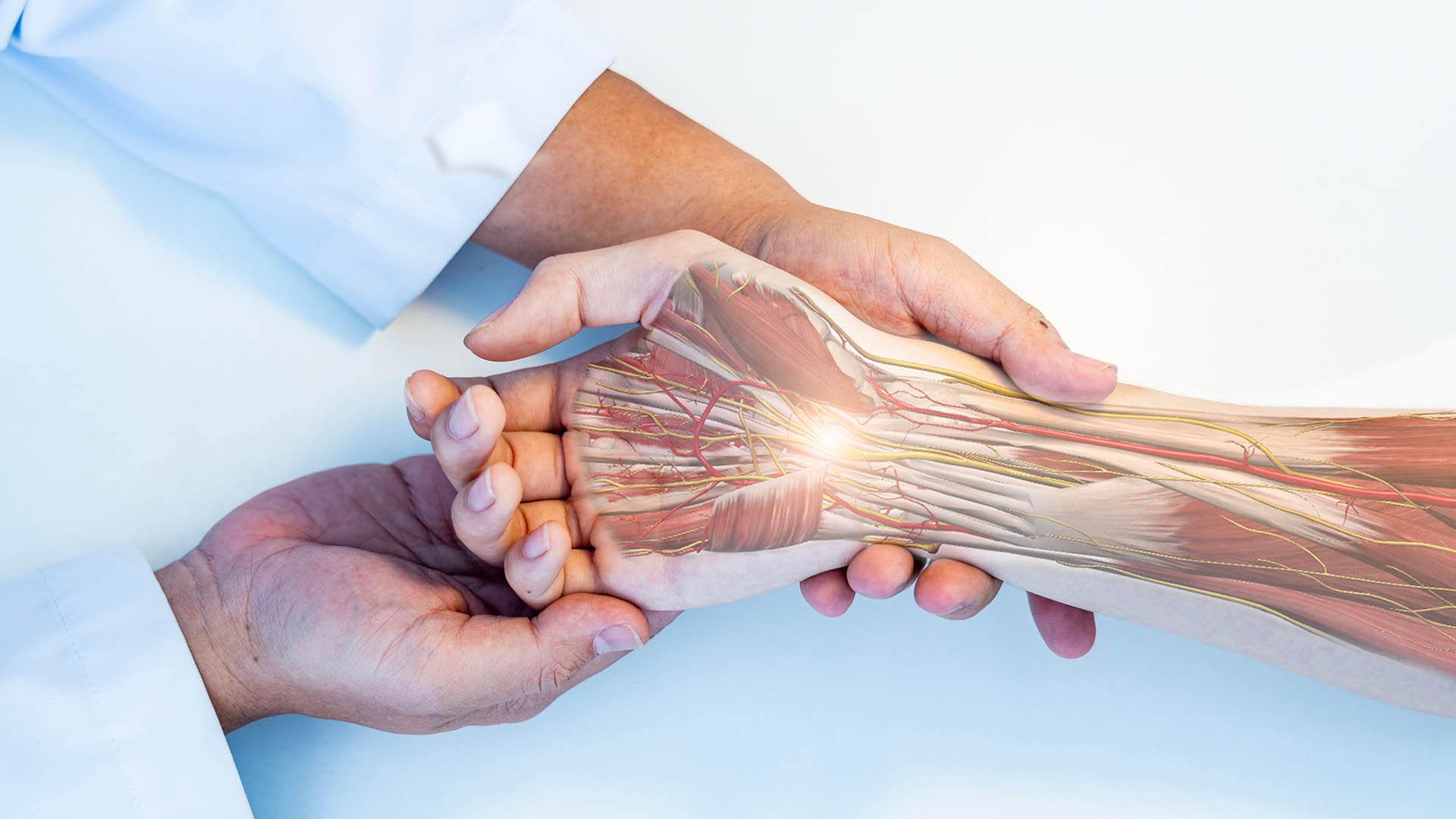
The Remplir device is designed to facilitate the repair of damaged peripheral nerves, an issue often resulting from traumatic injuries or surgical procedures. Remplir uses collagen-based scaffolding, which provides a bio-compatible framework that supports nerve regeneration. This technology encourages the natural reconnection of nerve fibres, promoting faster recovery and improved functional outcomes for patients.
Orthocell’s focus on Remplir aligns with the projected growth of the US nerve repair market, estimated to be worth over $1.6bn. The anticipated approval from the US Food and Drug Administration (FDA) could provide a significant boost to the company’s market share. Paul Anderson, Orthocell’s CEO, stated: “The funds raised will allow us to scale up manufacturing and distribution capacity, ensuring we can meet demand as we enter new markets”.
Beyond the US launch, Orthocell has made progress in other areas. The recent approval of Remplir in Singapore and its initial sales in Canada have contributed to two consecutive quarters of record revenue.
Orthocell’s portfolio also includes the Striate+ device, which has driven sales in dental bone regeneration.